Description
(Gray News) – Nasal spray products sold over the counter are being recalled after they were found to contain yeast and mold microbial contamination.
All lots of ReBoost Nasal Spray have been recalled as MediNatura New Mexico Inc. investigates the contamination, the Federal Drug Administration said in a statement.
MediNatura New Mexico Inc. is also recalling all lots of ClearLife Nasal Spray while the company investigates for microbial contamination that may be linked to high levels of Achromobacter.
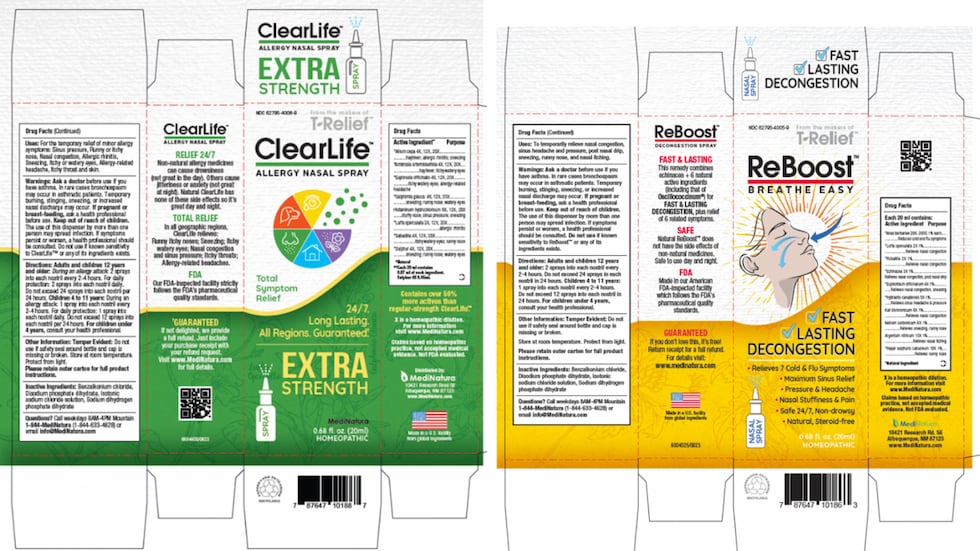
ReBoost Nasal Spray is used as a homeopathic nasal spray to temporarily relieve nasal congestion, sinus headache and pressure, postnasal drip, sneezing, runny nose and nasal itching and is packaged in a 20 mL bottle which is further packaged in a white and yellow carton.
The NDC number is 62795-4005-9 and the UPC: 787647 10186 3. Product expiration dates range from December 2022 to December 2025.
ClearLife Allergy Nasal Spray is used as a homeopathic nasal spray to temporarily relieve minor allergy symptoms such as sinus pressure, runny or itchy nose, nasal congestion, allergic rhinitis, sneezing, itchy or watery eyes, allergy-related headache and itchy throat and skin.
The product is packaged in a 20 mL bottle which is further packaged in a white and green carton.
The NDC number is 62795-4006-9 and the UPC: 787647 10188 7. Product expiration dates range from December 2022 to December 2025.
Using the recalled nasal sprays could cause adverse health effects, including life-threatening infections in people who are immunocompromised, the company said.
There have been no reports of illnesses related to this recall so far.
Anyone who has an adverse reaction to either of the products can file a report to the FDA’s MedWatch adverse event reporting program either online, by regular mail or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular mail or fax: download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Anyone with the recalled nasal sprays should stop using them immediately and return them to the place of purchase for a refund.
Copyright 2025 Gray Local Media, Inc. All rights reserved.
News Source : https://www.walb.com/2025/12/18/over-the-counter-nasal-sprays-recalled-potential-mold-contamination/
Other Related News
12/18/2025
VALDOSTA Ga WALB A domestic dispute call at a grocery store led to the arrest of a man wa...
12/18/2025
ALBANY Ga WALB One week ahead of Christmas the Today in Georgia TIG team added gifts unde...
12/18/2025
SALE CITY Ga WALB - A well-known dog-fighting breeder and trainer in Southwest Georgia was...
12/18/2025
WALB is working to produce a video for this story In the meantime we encourage you to watc...
12/18/2025







